45 what does open label mean
Open-label Definition & Meaning - Merriam-Webster open-la· bel ˌō-pən-ˈlā-bəl : being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject an open-label multicenter study compare double-blind, single-blind Word History First Known Use 1979, in the meaning defined above Time Traveler Open-Label Trial - an overview | ScienceDirect Topics An open-label trial has no blinding: everyone knows which patient is receiving which treatment. Open-label studies lack the rigor of blinded studies. ... Although the mean change was 10.1 points on the YMRS, the participants still had elevated mania scores at the end of the trial at 21.8. Other symptoms did improve, including depressive, ADHD ...
clinicalinfo.hiv.gov › en › glossaryOpen-Label Trial | NIH - HIV.gov Open-Label Trial. A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.

What does open label mean
What does an open date on a label mean? - Sage-Advices Open dating (calendar date as code) is stamped on a food product a product's package. It helps stores to determine time to display products, and the purchaser to know time limits to use the product at its best quality, not a safety date. Open dating is found on perishable foods: meat, poultry, eggs and dairy products. nckpharma.com › what-is-an-open-label-extension-studyWhat is an open label extension study? • NCK Pharma An open–label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. This contrasts with single blind and double blind experimental designs, where participants are not aware of what treatment they are receiving (researchers are also unaware in a double blind ... What does open-label mean? - definitions Definitions for open-label open-la·bel Here are all the possible meanings and translations of the word open-label. How to pronounce open-label? David US English Zira US English How to say open-label in sign language? Numerology Chaldean Numerology The numerical value of open-label in Chaldean Numerology is: 3 Pythagorean Numerology
What does open label mean. › understanding-clinical-trial-terminology-what-is-an-openUnderstanding Clinical Trial Terminology: What is an Open Label... Jun 24, 2019 · Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ... What Does Open Label Study Mean - October 2022 - Smartstartga.org What does open label study mean? An open label study is a research study in which the participants and investigators know what drug or treatment is being administered. In contrast, in a placebo-controlled study, the participants are not aware whether they are receiving the treatment or the placebo. › open-label › definitionMedical Definition of Open-label - MedicineNet Mar 29, 2021 · Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE. What does unblinded data mean? Explained by FAQ Blog Does open label mean unblinded? Open-label: A term used to explain the location when both the researcher and the player in a research learn about know the remedy the player is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what remedy the player is receiving.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1] Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc. What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are used to compare different treatments or gather further information on the long-term effects of new drugs and treatments. In some instances, patients are eligible to... PDF On-Label vs. Off-Label Prescribing - IG Living label doesn't mean FDA disapproves of an off-label use. Rather, the agency just hasn't reviewed that use. While prescribing off-label can be challenging for physicians, they have the discretion to do so; however, they are not free to promote the off-label use.5 Drugs that are commonly prescribed off-label include antide-
› content › 348What is an open label trial? | The BMJ May 23, 2014 · An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion envenomation, older than 6 months, and with no cardiorespiratory or central nervous system abnormalities. What Is 'Open-Box' and Should You Buy It? - dealnews Open-box products have a similarly bad reputation because the term suggests they're used. However, they may actually be brand-new items — just with a nice discount because the box happens to be open. While open-box products can be a good deal, that doesn't mean they always are. If you're considering buying open-box, here are a few things you ... Why an open banking white-label solution? | Klarna International Enter white label open banking services. But first up, what does "white label" mean? At its simplest, it refers to goods or services that are produced by one company, then purchased by another company to rebrand and sell as their own. Understanding Unapproved Use of Approved Drugs "Off Label" Unapproved use of an approved drug is often called "off-label" use. This term can mean that the drug is: Used for a disease or medical condition that it is not approved to treat, such as when ...
Open-label | definition of open-label by Medical dictionary open-label: (ō′pən-lā′bəl) adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo.
FDA-Approved Label vs. Off-Label: What Does It Mean? Per WebMD, "Off-label" means the medication is being used in a manner not specified in the FDA's approved packaging label, or insert." Why does the FDA allow off-label use? After the FDA approves a drug—which is an extensive process where the FDA evaluates the safety, effectiveness, and indication for a drug—healthcare providers can ...
Here's What 10 Symbols on Cosmetics Labels Mean | Mental Floss If the symbol is inside a solid circle, it means the packaging itself is made from recycled material. If the symbol is inside a circle and has a percentage inside the symbol or nearby, that...
Open-label Definition & Meaning | YourDictionary Open-label Definition Open-label Definition ōpən-lābəl Meanings Definition Source Adjective Filter adjective Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo. American Heritage Medicine Advertisement Find Similar Words
pubmed.ncbi.nlm.nih.gov › 17253876Open-label extension studies: do they provide meaningful ... - ... Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their ...
dian.wustl.edu › our-research › clinical-trialEnd of Trial and Open-Label Extension (OLE) Frequently Asked... What does "Open Label Extension or OLE" mean? Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given ...
Label Definition & Meaning - Merriam-Webster label 1 of 2 noun la· bel ˈlā-bəl 1 : a slip (as of paper or cloth) that is attached to something to identify or describe it 2 a : a descriptive or identifying word or phrase b : the brand name of a commercial product label 2 of 2 verb labeled or labelled; labeling or labelling -b (ə-)liŋ 1 : to attach a label to 2
Facebook - National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
What does each label mean? - Publisher Center Help - Google Labels are a set of predefined terms that describe the content of different parts of your news site and serve as hints to Google to help classify your content. We hope to provide useful ways for users to access the information they need despite the rapid changes in content creation worldwide. Sometimes, label application is informed by ...
What does open-label trial mean? - definitions.net An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered.
Open-label - definition of open-label by The Free Dictionary Define open-label. open-label synonyms, open-label pronunciation, open-label translation, English dictionary definition of open-label. adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a...
What does open-label mean? - definitions Definitions for open-label open-la·bel Here are all the possible meanings and translations of the word open-label. How to pronounce open-label? David US English Zira US English How to say open-label in sign language? Numerology Chaldean Numerology The numerical value of open-label in Chaldean Numerology is: 3 Pythagorean Numerology
nckpharma.com › what-is-an-open-label-extension-studyWhat is an open label extension study? • NCK Pharma An open–label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered. This contrasts with single blind and double blind experimental designs, where participants are not aware of what treatment they are receiving (researchers are also unaware in a double blind ...
What does an open date on a label mean? - Sage-Advices Open dating (calendar date as code) is stamped on a food product a product's package. It helps stores to determine time to display products, and the purchaser to know time limits to use the product at its best quality, not a safety date. Open dating is found on perishable foods: meat, poultry, eggs and dairy products.






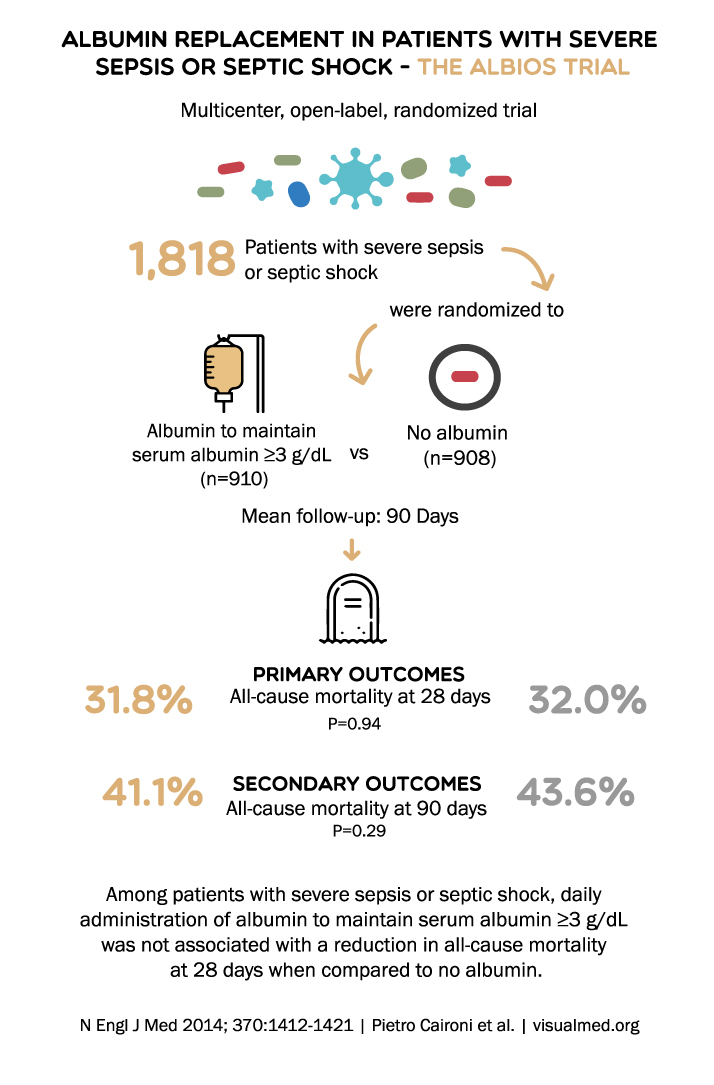










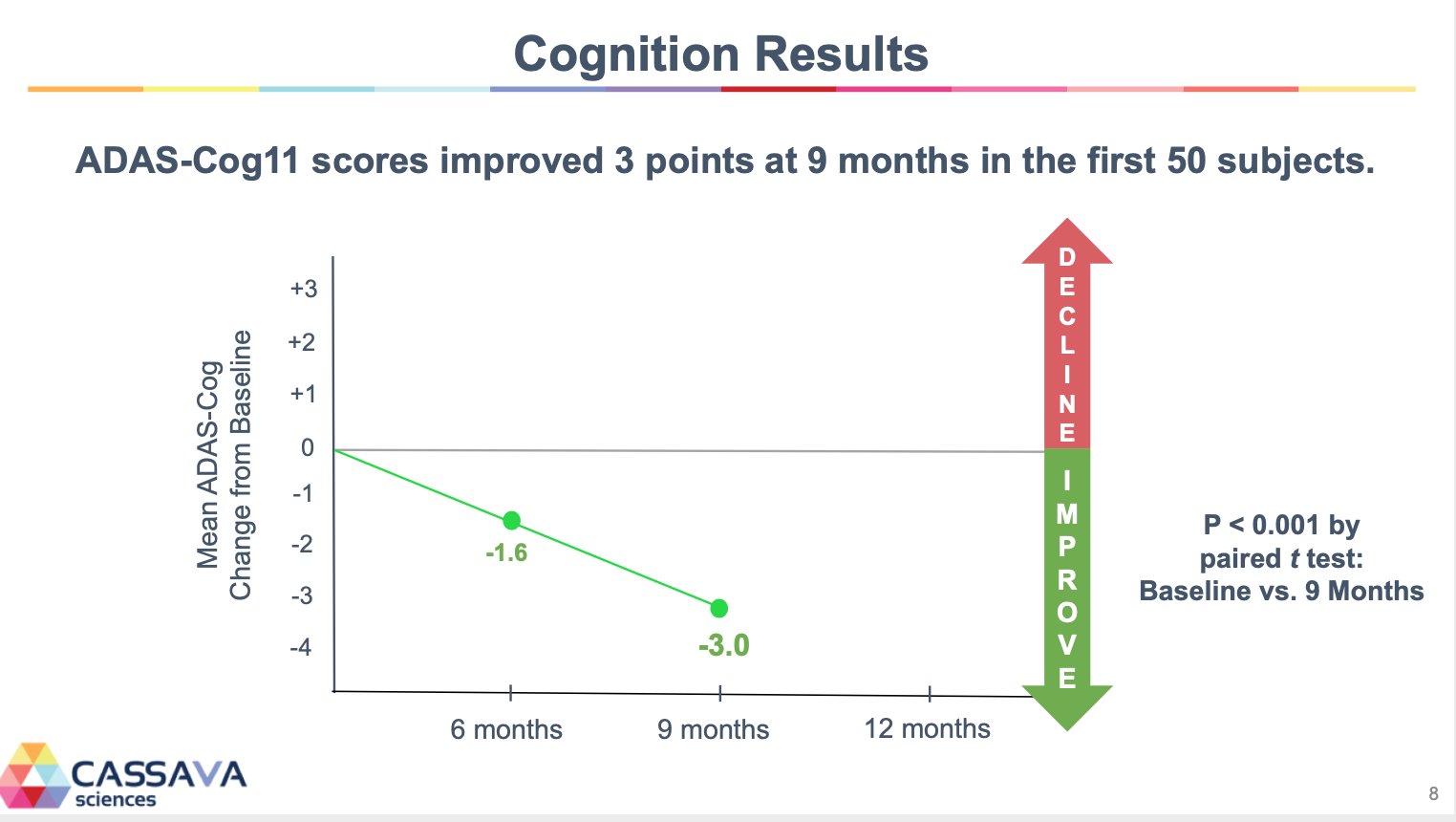
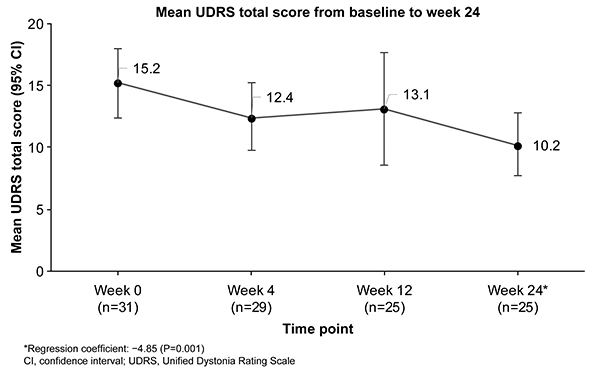





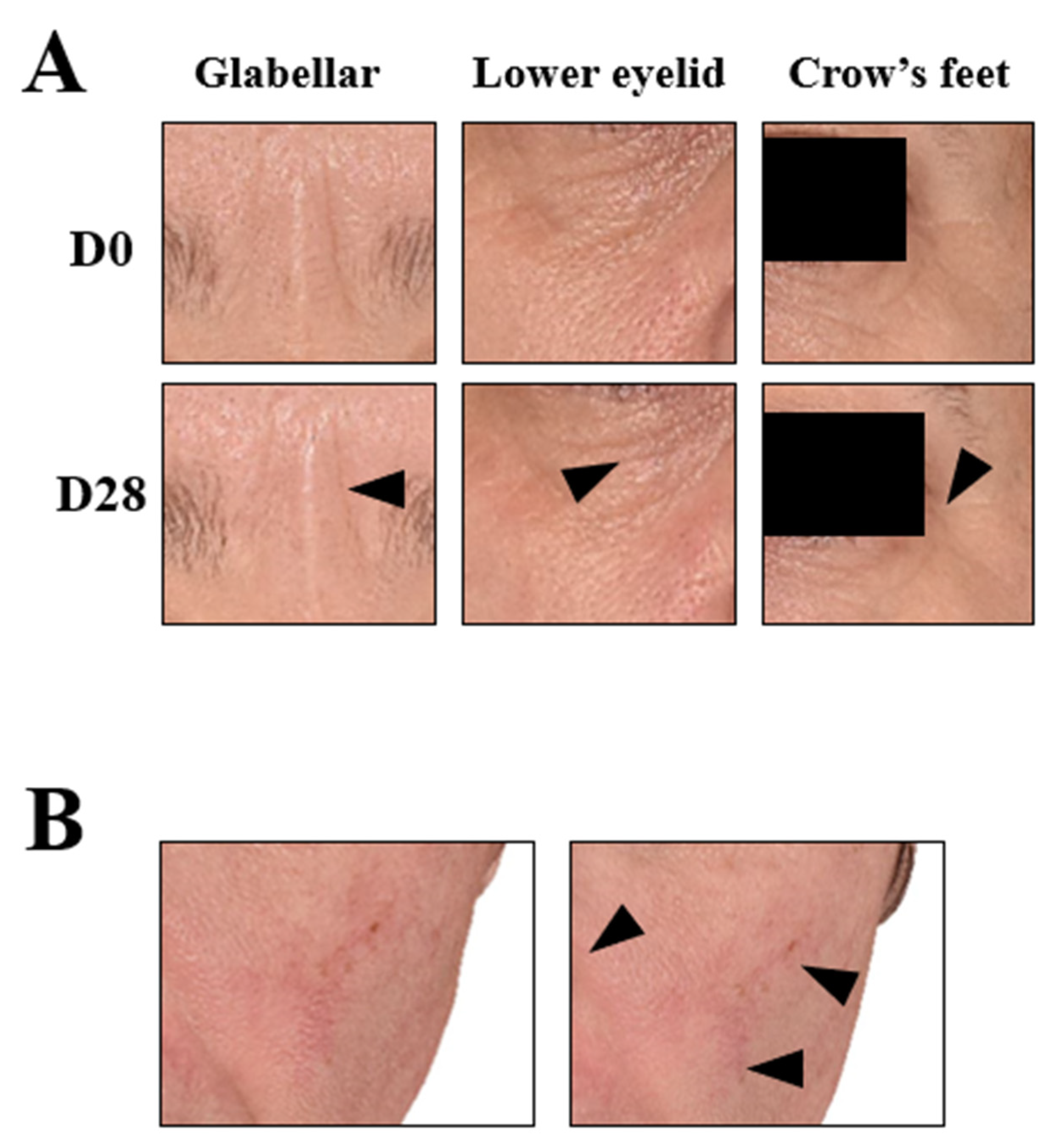

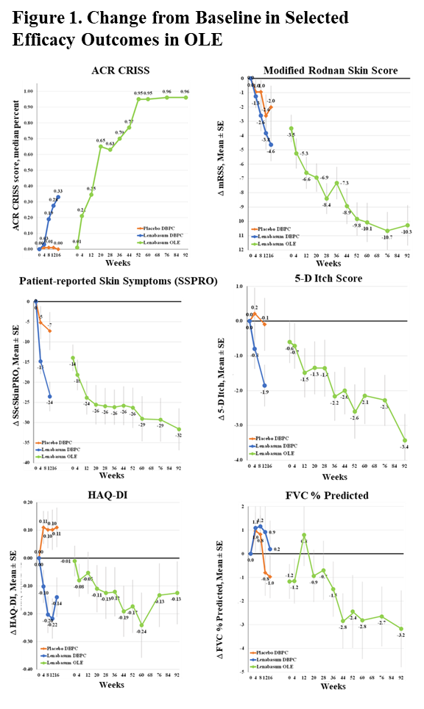
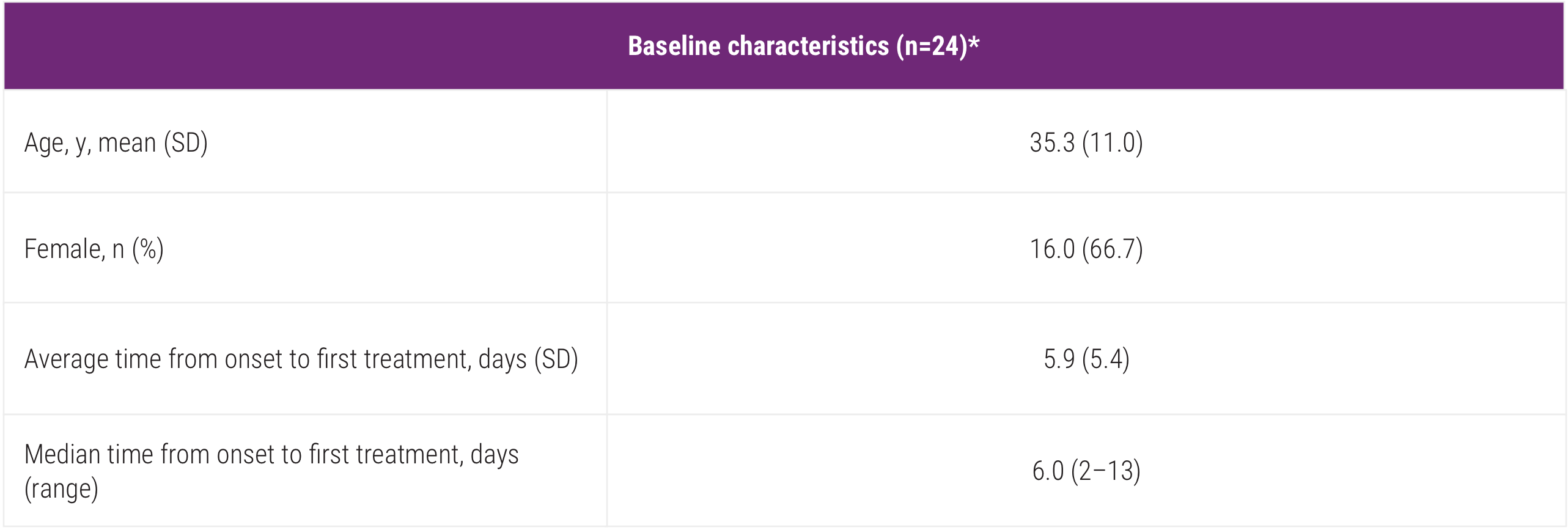



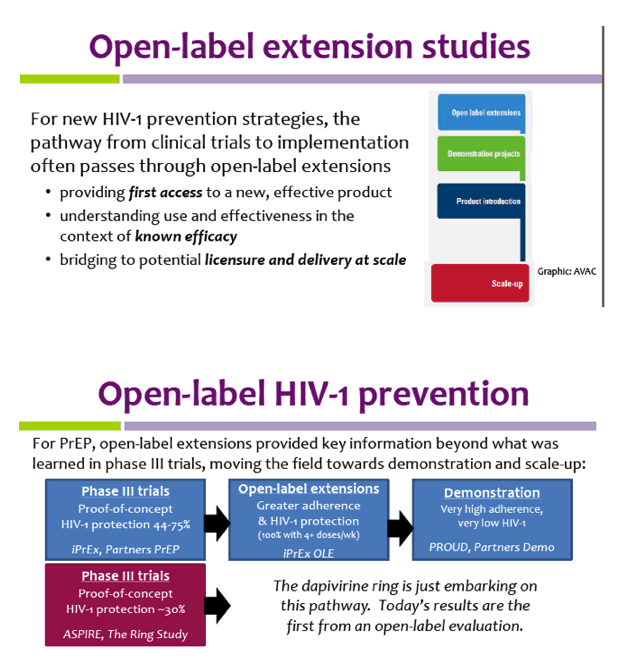

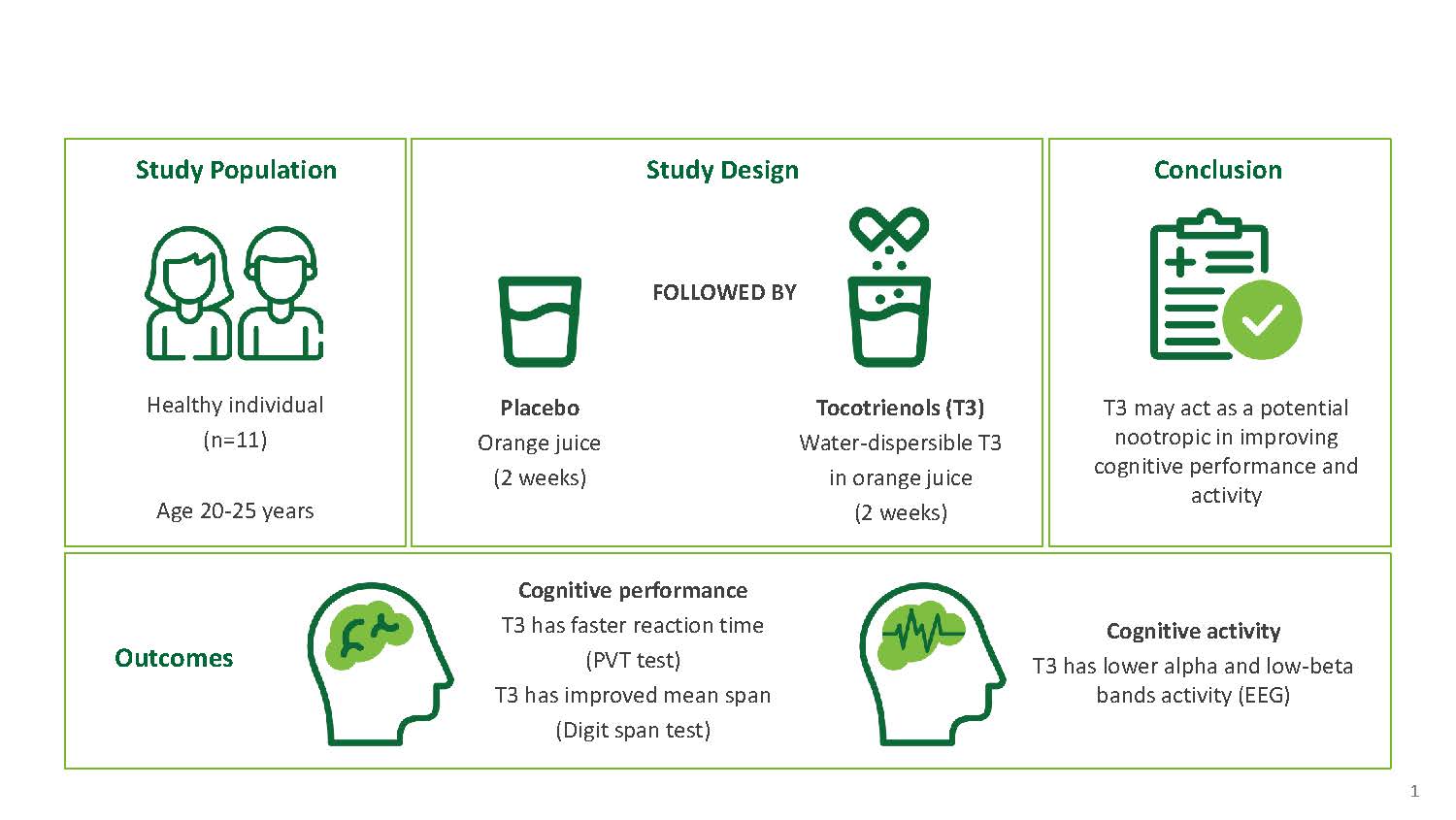



Post a Comment for "45 what does open label mean"